Sustainability
A global pioneer in the development of new drugs
Sustainable Partnerships
SK Biopharmaceuticals actively monitors and manages the sustainability of suppliers
based on our company’s internal policies regarding partner’s ESG risk management.
Management
SK Biopharmaceuticals conducts systematic reviews of our supply chain management policies and practices at the Board level to address potential ESG risks in the supply chain. We also obtain ESG guideline signatures from key suppliers based on our Partners’ ESG Management Policy. We manage communication channels and support trainings for our suppliers to promote sustainable partnerships and facilitate shared growth activities.
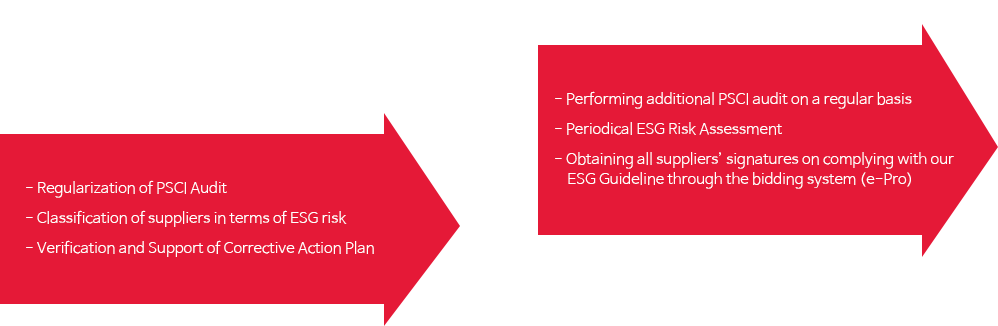
Targets
Performances
Participation in Initiatives to Manage Supply Chain Risks
In April 2022, SK Biopharmaceuticals became the first domestic pharmaceutical company to join the Pharmaceutical Supply Chain Initiative (PSCI). We are using the PSCI platform and database to examine histories and assess PSCI audit findings for our suppliers, and we eventually develop short-term and long-term PSCI audit strategies for our suppliers. Furthermore, we manage supply chain ESG risks proactively in accordance with PSCI's five domains: ethics, human rights and labor, health and safety, the environment, and management systems.ESG Risk Assessment and Audits
SK Biopharmaceuticals conducts ESG risk assessments on important suppliers every two years, in accordance with the Partners' ESG Management Policy, while consistently improving criteria and standards to match with worldwide norms. The risk of suppliers is assessed across four domains(Environment, Health and Safety, Labor and Human Rights, and Ethics).
Plan to Mitigate Supply Chain Risks
To compensate for potential supply chain risks, SK Biopharmaceuticals has devised and implemented a diversification strategy that includes numerous CMOs at various phases of the product manufacturing process. We are able to lessen our reliance on a single supplier through this supply chain diversification strategy, ensuring supply stability and the timely and ongoing distribution of products to fulfill global demand in the event of a supply disruption.Receiving the Pledge for the Practice of Ethics from Suppliers
SK Biopharmaceuticals seeks pledges for the Practice of Ethics from all suppliers, ensuring anti-corruption and compliance. One of the prerequisites for every potential supplier to register as our supplier and bid on our procurement platform is to sign the Practice of Ethics. Other vendors who do not use the platform are contacted directly to receive their pledge. SK Biopharmaceuticals has received pledge for the Practice of Ethics from domestic companies and will also receive it from foreign CMOs within 2023.Shared Growth Policy
SK Biopharmaceuticals is strengthening our collaboration with CMOs and material procurement partners,
and faithfully implementing four action policies.
1. Fair and Transparent Bidding
SK Biopharmaceuticals runs an electronic tender process in a fair and transparent way.2.Fair and Transparent Trading
SK Biopharmaceuticals is dedicated to maintaining fair and transparent trading practices in accordance with our procurement policies, while strengthening inspections by internal and external compliance teams.3.Training Support for Partners
SK Biopharmaceuticals provides our partners with various training opportunities aimed at creating and fostering a community for co-prosperity.4.Collaborative Tasks
SK Biopharmaceuticals has worked with partners to jointly develop projects from discovery to commercialization.Partnership ESG Management
SK Biopharmaceuticals works with partners in line with the quality
and safety standardsof global regulatory authorities such as the FDA and EMA.
Partners Management
SK Biopharmaceuticals has a code of conduct governing four areas (environment, health/safety, labor/human rights, and ethics) with 25 categories concerning partners management.How Partners are subjected to ESG Risk Management
SK Biopharmaceuticals identifies key partners based on the transaction amount, and requires partners to abide by the ESG guidelines whensigning new contracts.How and When ESG Risk Assessments and Due Diligence are Performed
SK Biopharmaceuticals conducts ESG risk assessment for partners every two years either by an external agency or through an in-house program.How Improvements are Requested and Supported
SK Biopharmaceuticals requests partners that need improvements to submit an improvement plan within 12 months. The company conducts due diligence to check whether they have properly executed the plan, and provides training and consulting in the following year after submission.Shared Growth Roadmap
SK Biopharmaceuticals has established a roadmap to achieve shared growth with our vendors
by the year 2030 and is faithfully committed to implementing it.
2024 : Establishment of Shared Growth Programs and Collaboration
Enhancing vendors' competitiveness through programs such as PSCI audit cost support for key managed companiesExpansion of education programs for vendors
2027 : Cultivating a Fair and Transparent Trading Culture, Expanding Shared Growth Programs, and Strengthening Collaboration
Strengthening internal and external compliance activities to establish a fair and transparent trading cultureExpanding Shared Growth programs for non-majored partners
Developing future pipelines with key vendors and collaborating to promote shared growth
2030 : Expansion of Joint Technology Development and Shared Growth Funds to Approximately 0.2% of Revenue
Supporting an increase in revenue for vendors through joint technology developmentExpanding the scale of education, welfare, and management support