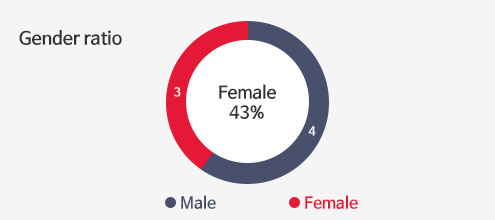
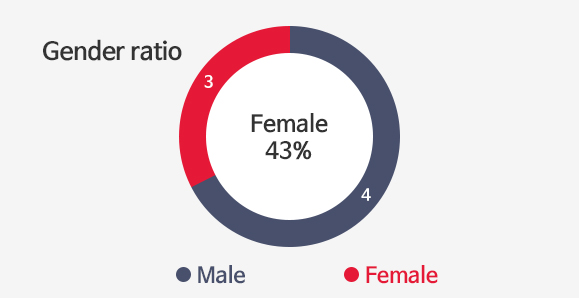
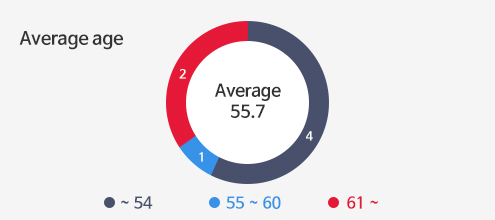
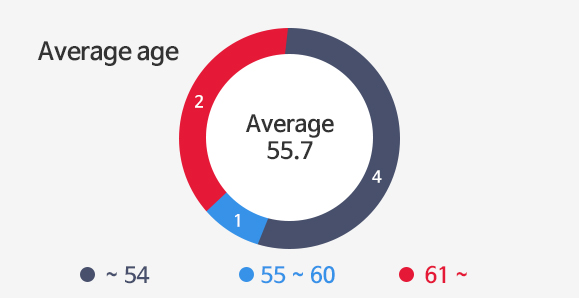
Investors
A global pioneer in the development of new drugs
Composition
Executive Director
-

Lee Dong-HoonCEO/President
Appointment Date2023.03.28
Term2023.03. ~ 2026.03.
Career(Present) CEO of SK Biopharmaceuticals,
CEO of SK Life Science, Inc.,
CEO of SK Life Science Labs, Inc.
-

Jung Ji-YoungDirector
Appointment Date2024.03.26.
Term2024.03. ~ 2027.03.
Career(Present) CFO of SK Biopharmaceuticals
(Former) Head of Corporate Finance of SK Biopharmaceuticals
Non-executive Director
-

Kim Yeon-TaeDirector
Appointment Date2023.03.28
Term2023.03. ~ 2026.03.
Career(Present) Excutive Vice President of Head of SK Bio Investment
(Former) Director of SK Bio Investment Center
Independent Director
-

Suh Ji-HeeDirector
Appointment Date2024.03.26.
Term2024.03. ~ 2027.03.
Career(Present) Special Professor of School of Business at Ewha Womans University
(Present) Vice President of KICPA
(Former) Vice President of KPMG Samjong Accounting Corp.
-

Kim Min-JiDirector
Appointment Date2023.03.28.
Term2023.03. ~ 2026.03.
Career(Former) CBO of Mineralys Therapeutics, Inc.
(Former) AffaMed Digital, General Manager
-

Kim Yong-JinDirector
Appointment Date2025.03
Term2025.03 ~ 2028.03
Career(Present) Professor at Seoul National University College of Medicine
(Present) Director of Life Research Institute at Seoul National University Hospital
-

Cho Kyung-SunDirector
Appointment Date2025.03
Term2025.03 ~ 2028.03
Career(Present) Shinhan DS Standing Adviser
(Former) CEO of Shinhan DS
(Former) Vice President, Digital Individual Group, Shinhan Bank
Introducing Board Skills Matrix (BSM)
The SK Biopharmaceuticals Board of Directors responds to the needs of various stakeholders, including shareholders, investors,
and the market, by introducing the BSM. The BSM is a data that visually organizes and displays information on the capabilities,
qualities, and diversity of the members of the BOD, which will increase stakeholders' understanding of the composition of the
BOD, increase the efficiency of the operation of the BOD, and strengthen the transparency of governance.
-
Leadership
Expertise in large-scale
organizational operations
-
International Relationship/
Global BusinessExpertise in international
relationship, regional risk and
global business
-
M&A/Capital markets
Expertise in M&A, financing
the investment activities of the
company
-
Industry
Expertise in the company's core
industries, such as pharmaceuticals,
licenses and permits
-
Finance/Accounting and Risk
Expertise in finance and
accounting for the management
and supervision of the company
-
Legal/Public policy
Expertise in legal risk and public
policy in the management of
the company
-
ESG
Expertise in environment,
society, and governance
-
R&D/Tech.
Expertise in the field of science
and high technology
Activities of Board of Directors
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 01.09. | Establishment of JV between SK Biopharmaceuticals and Eurofarma | Approved | 100% |
| 2nd | 02.06. | Approval of Financial Statements for the 14th term (2024) | Approved | 100% |
| Approval of the Business Report for the 14th term (2024) | Approved | |||
| The Service Agreement with SK Biotek Co., Ltd. | Approved | |||
| 2024 Report on the Operational Status of Internal Accounting Management System | Reported | |||
| Revision of Internal Accounting Management System Regulation | Reported | |||
| Report on KPI Results in 2024 | Reported | |||
| 2025 Results of Director Remuneration Limits Review | Reported | |||
| 3rd | 03.06. | Convocation of the 14th Ordinary General Meeting of Shareholders and Confirmation of Agenda. | Approved | 100% |
| Purchasing the PINX membership | Approved | |||
| 2024 Evaluation of Operational Status of Internal Accounting Management System | Reported | |||
| Operational Status of Compliance Control Standards for 2024 | Reported | |||
| 2024 Operational Evaluation of the Board of Directors and Committees | Reported | |||
| 4th | 03.20. | Change of Service Contract with SK Biotek Co., Ltd. | Approved | 100% |
| Carisbamate Development Status Report | Reported | |||
| 5th | 03.26. | Appointment of the chairperson of the board of directors | Approved | 100% |
| Composition of the Committee and appointment of its members | Approved | |||
| 6th | 04.17. | SUPEX Council contribution transaction | Approved | 100% |
| Purchase transactions of Commercial Drug Substances with SK Biotek Co., Ltd. | Approved | |||
| 2025 standard renewal service transaction with SK Biotek Co., Ltd. | Approved | |||
| Service transaction with SK Biotek Co., Ltd. for the supply of raw materials drugs from Japanese partner company | Approved | |||
| Deploy SAP ERP Upgrade with SK Inc. | Approved | |||
| 2024 Decision of Directors Remuneration | Approved | |||
| 7th | 05.09. | Signing a contract to change mySUNI with SK Innovation | Approved | 100% |
| Service transaction with Biotek Co.,Ltd. to support the registration and supply of raw materials drugs from Chinese partner company | Approved | |||
| Renewal of Directors & Officers Liability Insurance (D&O insurance) | Approved | |||
| Report of Business Performance for the Q1 2025 | Reported | |||
| 8th | 06.10. | Research Collaboration Agreement Signing with SK Life Science, Inc. | Approved | 100% |
| Revision of the Audit Committee Regulations | Approved | |||
| Selection of Key ESG Issues for SK Biopharmaceuticals in 2025 | Reported | |||
| Publication of SK Biopharmaceutical's Sustainable Management Report for 2025 | Reported | |||
| 9th | 07.08. | Project Agreement for Office Security Infrastructure Upgrade and Deployment with SK Inc. | Approved | 100% |
| 2025 KPI Changes | Reported | |||
| 10th | 08.05. | Signing of an Agreement with SK Inc. for the Introduction of the A.Biz Service | Approved | 100% |
| The Service Agreement with SK Biotek Co., Ltd. | Approved | |||
| Report of Business Performance for the Q2 2025 | Reported | |||
| 11th | 09.23. | Contract with SK Inc. for the Establishment of a Retirement Pension Program | Approved | 100% |
| 12th | 10.27. | Review Results on the CEO Retention | Reported | 100% |
| 13th | 11.05. | The Service Agreement with SK Biotek Co., Ltd. | Approved | 100% |
| Contract with SK Inc. for the Functional Enhancement of the Expense Settlement System | Approved | |||
| Report of Business Performance for the Q3 2025 | Reported |
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 01.04. | Export of Cenobamate technology to Korea and RoW region | Approved | 100% |
| Updates on Open Innovation Fund (LifeSci) Investment | Approved | |||
| Agreement to change License for bulk tablet production to Angelini | Reported | |||
| 2nd | 01.29. | Approval of Internal Directors Remuneration in 2024 | Approved | 100% |
| Report on KPI Results in 2023 | Reported | |||
| 3rd | 02.05. | Approval of Financial Statements for the 13th term (2023) | Approved | 100% |
| Approval of the Business Report for the 13th term (2023) | Approved | |||
| Purchasing the PINX membership | Approved | |||
| Power Purchase Agreement with SK E&S | Approved | |||
| Appointment of Compliance Assistant | Approved | |||
| 2024 KPI Establishment | Reported | |||
| 2024 Report on the Results of Director Remuneration Limits Review | Reported | |||
| 2023 Report on the Operational Status of Internal Accounting Management System | Reported | |||
| Report on the Operational Status of Compliance Control Standards for 2023 | Reported | |||
| 4th | 03.07. | Convocation of the 13th Ordinary General Meeting of Shareholders and Confirmation of Agenda. | Approved | 100% |
| 2023 Finalization of Report on Evaluation of Operational Status of Internal Accounting Management System | Reported | |||
| 5th | 03.26. | Revision of Executive Management Regulation | Approved | 100% |
| Abolition of the Governance Committee and Establishment of the Independent Directors’ Council | Approved | |||
| Amendment of the Corporate Governance Charter and the BOD and Committees Regulations | Approved | |||
| Composition of the Committee and appointment of its members | Approved | |||
| 2023 Operational Evaluation of the Board of Directors and Committees | Reported | |||
| 6th | 04.18. | SUPEX Council contribution transaction | Approved | 100% |
| License-out of Non-opioid Pain Drug Candidates | Approved | |||
| 2024 CEO Long-term Incentive | Approved | |||
| 2024 Decision of Directors Remuneration | Approved | |||
| 7th | 05.09. | Renewal of Directors & Officers Liability Insurance (D&O insurance) | Approved | 100% |
| System Construction Agreement with SK Inc. | Approved | |||
| Report on the Disclosure of the 2024 Corporate Governance Report | Reported | |||
| Report of Business Performance for the Q1 2024 | Reported | |||
| Selection of Key ESG Issues for SK Biopharmaceuticals in 2024 | Reported | |||
| 8th | 06.20. | Purchase of Commercial Drug Substances with SK Biotek Co., Ltd. | Approved | 100% |
| Regulations on Compensation for Independent Directors | Reported | |||
| Publication of SK Biopharmaceutical's Sustainable Management Report for 2024 | Reported | |||
| 9th | 07.17. | Licensing Agreement for Radioactive Drug Candidate | Approved | 100% |
| AWS Cloud Infrastructure Operation Agreement with SK Inc. | Approved | |||
| 10th | 08.08. | Securing the Right to Business Negotiation 225Ac with TerraPower | Approved | 100% |
| Report of Business Performance for the Q2 2024 | Reported | |||
| 11th | 09.25. | M365 Upgrade Deployment with SK Inc. | Approved | 100% |
| 12th | 11.08. | Report of Business Performance for the Q3 2024 | Reported | 100% |
| 13th | 11.28. | Request for re-approval of transactions with the SUPEX Council (Change of Party) | Approved | 100% |
| Request for approval of renewing the basic transaction with SK Biotek Co., Ltd. | Approved | |||
| The Service Agreement with SK Biotek Co., Ltd. | Approved | |||
| Interim Report on the KPI Structure for 2025 | Reported | |||
| Result of Trainings (DE&I / Human rights / Disability Awareness / Prevention of Sexual Harrassment) | Reported | |||
| Results of CEO evaluation and Retention Review | Reported | |||
| 14th | 12.12. | Approval of Internal Directors Remuneration in 2025 | Approved | 100% |
| Renewal of the Consignment Contract on Management of Information System with SK Inc. | Approved | |||
| Fees for the Consignment Contract on Management of Information System with SK Inc. | Approved | |||
| Request for approval of mySUNI-related transaction with SK Innovation | Approved | |||
| Commodity transactions with SK Life Science, Inc. | Approved | |||
| Service Transactions with SK Life Science, Inc. | Approved | |||
| Service Transactions with SK Biopharmaceutical Science and Technology (Shanghai) | Approved | |||
| Service Transactions with SK Life Science Labs, Inc. | Approved | |||
| Report on the KPI Structure for 2025 | Reported | |||
| Short-Term Management Plan for 15th Term (2025) | Reported | |||
| 2025 Reorganization and Appointment of executives | Reported |
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 02.09. | Approval of Financial Statements for the 12th term (2022). | Approved | 80% |
| Approval of the Business Report for the 12th term (2022). | Approved | |||
| Credit Limit Management System Development Agreement with SK Co., Ltd. | Approved | |||
| Purchasing the PINX membership | Approved | |||
| 2022 KPI Performance Rating | Reported | |||
| 2022 Report on the Operational Status of Internal Accounting Management System | Reported | |||
| Selection of 2023 Key Agenda for Sustainable Management | Reported | |||
| Report on the Operational Status of Compliance Control Standards for 2022. | Reported | |||
| 2nd | 03.09. | Approval of Internal Directors Remuneration in 2022. | Approved | 80% |
| Convocation of the 12th Ordinary General Meeting of Shareholders and Confirmation of Agenda. | Approved | |||
| 2023 Report on the Results of Director Remuneration Limits Review | Reported | |||
| 2022 Finalization of Report on Evaluation of Operational Status of Internal Accounting Management System | Reported | |||
| 2022 Operational Evaluation of the Board of Directors and Committees | Reported | |||
| 3rd | 03.28. | Election of CEO | Approved | 100% |
| Composition of the Committee and appointment of its members | Approved | |||
| Reorganization and executive shuffling. | Reported | |||
| 4th | 04.27. | Appointment of the chairperson of the board of directors | Approved | 100% |
| Adjustment of transaction limit due to recalculation of brand royalties with SK Co., Ltd. | Approved | |||
| Software Development Agreement with SK Co., Ltd. | Approved | |||
| SUPEX Council contribution transaction | Approved | |||
| 2023 KPI Establishment | Reported | |||
| 5th | 05.11. | Investment in Open Innovation Fund (LifeSci) | Approved | 100% |
| Renewal of Directors & Officers Liability Insurance (D&O insurance) | Approved | |||
| Appointment of Compliance Assistant | Approved | |||
| Report of Business Performance for the Q1 2023 | Reported | |||
| 6th | 06.29. | Investment in the acquisition of Proteovant | Approved | 100% |
| Liquidation of Kinisi Therapeutics and withdrawal of Relenopride License | Approved | |||
| Appointment of Compliance Assistant | Approved | |||
| Cala Health Insider Round Investment | Reported | |||
| 7th | 07.20. | Case of increasing the limit of product transaction with SK Life Science, Inc. | Approved | 100% |
| CEO Compensation Plan | Approved | |||
| Transaction of contributions to the AI subcommittee under the ICT Committee | Reported | |||
| 2023 Financial Story | Reported | |||
| Publication of SK Biopharmaceutical's Sustainable Management Report for 2023 | Reported | |||
| 8th | 08.17. | Signed MENA regional strategic alliance partnership agreement and Cenobamate technology export agreement | Approved | 100% |
| Report of Business Performance for the Q2 2023 | Reported | |||
| 9th | 09.14. | Report on the 2023 Human Rights policy | Reported | 100% |
| Report on the training results (DE&I / Human rights / Disability awareness improvement / Sexual harassment prevention) | Reported | |||
| Report on the 2023 Compliance Survey result and enhancement measures | Reported | |||
| 10th | 09.26. | Shared Service Provision Plan to Proteovant | Approved | 100% |
| 11st | 11.09. | R&D Collaboration Agreement with SK Life Science Labs | Approved | 100% |
| Audit Agreement with SK Life Science Inc. | Approved | |||
| Report of Business Performance for the Q3 2023 | Reported | |||
| Report on the result of Culture Survey / Human Right Survey 2023 | Reported | |||
| Report on Reorganization of Global function | Reported | |||
| Report on Status of Partnering | Reported | |||
| 12th | 12.05. | 2024 Reorganization and Appointment of executives | Reported | 100% |
| 13th | 12.14. | Commodity transactions with SK Life Science, Inc. | Approved | 100% |
| Service Transactions with SK Life Science, Inc. | Approved | |||
| Service transactions with SK Biopharmaceutical Science and Technology (Shanghai) | Approved | |||
| Transactions for entrusting information system management business with SK Inc. | Approved | |||
| Brand royalties Transactions with SK Inc. | Approved | |||
| Approval of limits on large-scale insider trading for 2024 | Approved | |||
| Report on Short-Term Management Plan for 14th Term (2024) | Reported |
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 02.08. | Approval of Financial Statements for the 11th term (2021). | Approved | 100% |
| Approval of the Business Report for the 11th term (2021). | Approved | |||
| Amendment of the SUPEX Council Covenant and Agreement | Approved | |||
| Increase of the Limit Following the Recalculation of Brand Royalty with SK Co., Ltd. | Approved | |||
| Approval of Internal Director Remuneration in 2022. | Approved | |||
| 2021 KPI Performance Rating. | Reported | |||
| 2021 Report on the Operational Status of the Internal Accounting Management System | Reported | |||
| 2022 Report on the Results of Director Remuneration Limits Review | Reported | |||
| 2022 Report on the Results of Independent Director Re-appointment Review | Reported | |||
| 2nd | 03.08. | Approval of Final Version of Financial Statements for the 11th term (2021) | Approved | 100% |
| Convocation of the 11th Ordinary General Meeting of Shareholders and Confirmation of Agenda. | Approved | |||
| Implementation Agreement with SK for Security Settlement System | Approved | |||
| Purchase of Finx membership | Approved | |||
| 2021 Finalization of Report on Evaluation of Operational Status of Internal Accounting Management System | Reported | |||
| 2021 Report on the Operational Status of the Compliance Control Standards | Reported | |||
| 2021 Report on the Board of Directors Operational Evaluation | Reported | |||
| 3rd | 03.24. | Election of the Chairman of the Board of Directors. | Approved | 100% |
| Election of CEO | Approved | |||
| Composition and appointment of members of the Committee | Approved | |||
| The Execution of Directors' Remuneration | Approved | |||
| 2022 KPI Establishment | Reported | |||
| 4th | 04.21. | SUPEX Council Contribution Transactions | Approved | 100% |
| Amendment of the Board of Directors regulations | Approved | |||
| Amendment of regulations of the Committee | Approved | |||
| 5th | 05.12. | Approval of the amount of research service transactions with SK Biotek Co., Ltd. | Approved | 100% |
| Renewal of Directors & Officers Liability Insurance (D&O insurance) | Approved | |||
| Report of Business Performance for the Q1 2022 | Reported | |||
| Export of Cenobamate Technology to Israel | Reported | |||
| 6th | 06.16. | Contract for Introduction of Integrated Certification System with SK Co., Ltd | Reported | 100% |
| Report on Support for Limited Loan Agreements for SK Life Science, Inc. | Reported | |||
| Transaction of contributions to the AI subcommittee under the ICT Committee | Reported | |||
| Publication of SK Biopharmaceutical's Sustainable Management Report for 2022 | Reported | |||
| 7th | 07.14. | Approval for the purchase of commercial drug substances with SK Biotek Co., Ltd. | Approved | 80% |
| Approval of limits on large-scale insider trading in Q3 2022 | Approved | |||
| Signed a long-term loan agreement with NH Nonghyup Bank | Approved | |||
| Export Cenobamate technology to LATAM (Latin America) | Approved | |||
| 8th | 08.11. | Report on Business Performance for Q2 2022 | Reported | 100% |
| Report on the status of litigation related to SK LSI clinical trials | Reported | |||
| 9th | 09.29. | Approval of signing a research cooperation contract with SK Life Science, Inc. | Approved | 100% |
| Revision of Audit Committee Regulations | Approved | |||
| Ethical management level measurement system | Reported | |||
| 10th | 10.28. | Report on pending issues | Reported | 100% |
| 11th | 11.10. | Renewal of commercialization supply contract with SK Biotek Co., Ltd. and order for drug substance purchase | Approved | 80% |
| Change of Commercialization Service Agreement between SKBP-SKLSI | Approved | |||
| Report on Business Performance for Q3 2022 | Reported | |||
| Settlement of lawsuits for damages in connection with the sale of Arvelle's shares | Reported | |||
| 12th | 11.30. | Introduction of Board Skils Matrix | Approved | 100% |
| Re-appointment and job assignments of operational executive officers | Reported | |||
| 13th | 12.15. | Commodity transactions with SK Life Science, Inc. | Approved | 80% |
| Service Transactions with SK Life Science, Inc. | Approved | |||
| Service transactions with SK Biopharmaceutical Science and Technology (Shanghai corporation) | Approved | |||
| Transactions for entrusting information system management business with SK Corporation | Approved | |||
| Approval of limits on large-scale insider trading for 2023 | Approved | |||
| Approval of changes related to Ignis investment | Approved | |||
| Establishment of Short-Term Management Plan for the 13th Term (2023) | Reported |
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 01.04. | Concluding a stock purchase agreement with the Avelle | Approved | 100% |
| 2nd | 02.08. | Approval of financial statements for the 10th period (2020) | Approved | 100% |
| Approval of the business report for the 10th term (2020) | Approved | |||
| Resolution of the short-term management plan for the 11th term (2021) | Approved | |||
| Introduction of the electronic voting system at the general meeting of shareholders | Approved | |||
| Report on the operational status of the internal accounting management system in 2020 | Reported | |||
| Report on the financial statements of the 10th term (2020) | Reported | |||
| 3rd | 03.03. | Proposal of agenda items of the Ordinary General Meeting of Shareholders concerning the grant of stock options | Approved | 100% |
| Proposal of agenda items at the Ordinary General Meeting of Shareholders for partial amendments to the articles of incorporation | Approved | |||
| Convocation of the 10th Ordinary General Meeting of Shareholders and confirmation of agenda | Approved | |||
| Asset transaction with SK Finx | Approved | |||
| Report on the results of the evaluation of the operational status of the internal accounting management system in 2020 | Reported | |||
| Report on the actual status of the operation of compliance control standards | Reported | |||
| 4th | 04.21. | Appointment of the chairperson of the board of directors | Approved | 100% |
| Establishment of a corporate governance charter | Approved | |||
| Amendment of the regulations of the board of directors | Approved | |||
| Amendment of the regulations of the Committee (Audit Committee, Governance Committee) | Approved | |||
| Establishment of the ESG/Strategic Committee and appointment of the members of the ESG/Strategic Committee | Approved | |||
| Enactment of regulations on the ESG/Strategic Committee | Approved | |||
| Establishment of the Nomination and Compensation committee and appointment of personnel affairs | Approved | |||
| Enactment of regulations of the Nomination and Compensation Committee | Approved | |||
| Transaction of contributions to the SUPEX Pursuit Council | Approved | |||
| A contract for the establishment of an integrated management information system with the SK Holdings Co | Approved | |||
| 5th | 05.12. | Determination of individual remuneration for inside directors in '21 | Approved | 100% |
| Renewal of executive liability insurance (D&O insurance) | Approved | |||
| Establishment of an annual target (KPI) | Reported | |||
| Report on business performance for the second quarter of 2021 | Reported | |||
| 6th | 06.29. | Contributions to the AI Subcommittee under the ICT Committee | Approved | 100% |
| SAP Software License Agreement with SK Inc. | Approved | |||
| Commercial transaction with SK Life Science Inc. | Approved | |||
| 2021 Sustainability Report | Approved | |||
| 2021 Financial Story | Reported | |||
| 7th | 08.10. | Contract for shared Development of Group Consolidated Internal Accounting Management System with SK Inc. | Approved | 100% |
| Amendment of the Guidelines for Practicing Code of Ethics and Enactment of the Code of Conduct for Anti-Corruption | Approved | |||
| Concluding Long-term loan agreement with a bank | Approved | |||
| Report of Business Performance for the First Half of 2021 | Reported | |||
| Status on Open Innovation Progress | Reported | |||
| 8th | 11.11. | Out-licensing in China and establishment of a new corporation | Approved | 100% |
| Commercial drug substance purchase contract with SK Biotek Co.,Ltd | Approved | |||
| Contract for Process Validation(PV) Preparation Service with SK Biotek Co.,Ltd | Approved | |||
| Report of Business Performance for the third quarter of 2021 | Reported | |||
| Report on Legal issues | Reported | |||
| 9th | 12.01. | Consignment transaction for information system management with SK Co., Ltd | Approved | 100% |
| Product transaction with SK Life Science, Inc. | Approved | |||
| Service transaction with SK Life Science, Inc. | Approved | |||
| Service transaction with SK Biopharm Tech (Shanghai) | Approved | |||
| Approval of Large-scale internal transaction limit in 2022 | Approved | |||
| Report of the short-term management plan for the 12th term (2022) | Reported | |||
| Assignment of duties, appointment, and retirement of the Executive | Reported | |||
| 10th | 12.23. | Out-licensing of cenobamate in Canada | Approved | 100% |
| Round | Date Held | Agenda | Results of Deliberation |
Director Participation |
|---|---|---|---|---|
| 1st | 02.06. | Finalized financial statements for the 9th term | Approved | 100% |
| Approved sales reports for the 9th term | Approved | |||
| Resolved short-term management plan for the 10th term | Approved | |||
| Called and finalized agenda for the 1st special meeting of shareholders in 2020 | Approved | |||
| Designated shareholder list closing and reference dates | Approved | |||
| Signed clinical drug substance supply contract with SK Biotek | Approved | |||
| 2nd | 03.05. | Appointed the Chair of the Board of Directors | Approved | 100% |
| Introduced agenda for 9th regular general meeting of shareholders about revising the Articles of Incorporation | Approved | |||
| Revised the Board of Directors regulation | Approved | |||
| Revised board member management regulation | Approved | |||
| Called and finalized agenda for the regular general meeting of shareholders | Approved | |||
| 3rd | 03.27. | Service transactions with SK Life Science, Inc. | Approved | 100% |
| Product transactions with SK Life Science, Inc. | Approved | |||
| Service transactions with SK Biopharm Tech (Shanghai) | Approved | |||
| Signed basic contract with SK Biotek | Approved | |||
| Approved concurrent office of directors | Approved | |||
| Schedule for the listing | Reported | |||
| 4th | 04.23. | Appointed Board of Directors Chair | Approved | 100% |
| Approved quarterly transaction amount (limit) with SK Biotek | Approved | |||
| Information system management entrustment contract with SK | Approved | |||
| Stage gate system improvement contract with SK | Approved | |||
| Animal experiment resource management system contract with SK | Approved | |||
| Research cooperation contract with SK Life Science, Inc. | Approved | |||
| Revision of the internal accounting management regulation | Reported | |||
| Schedule for the listing | Reported | |||
| 5th | 05.19. | Issued new shares and approved secondary distribution to be listed on the securities market | Approved | 100% |
| Directors and officers liability insurance | Reported | |||
| 6th | 06.19. | Finalized share-issuing conditions to be listed on the securities market | Approved | 80% |
| Appointed compliance assistant | Approved | |||
| Established compliance control criteria | Approved | |||
| Software (Microsoft) license contract with SK | Approved | |||
| Software (SAP) license contract with SK | Approved | |||
| Network environment contract with SK | Approved | |||
| Cenobamate process service contract with SK Biotek | Approved | |||
| 7th | 08.13. | Service transactions with SK Biotek | Approved | 100% |
| Commercial drug substance purchase contract with SK Biotek | Approved | |||
| Legal system contract with SK | Approved | |||
| Report on business performance for the first half of 2020 | Reported | |||
| 8th | 10.13. | Signed regional development and commercialization license-out contract for Cenobamate in Japan | Approved | 100% |
| Investment in Open Innovation Fund | Reported | |||
| 9th | 12.17. | Participated as partner in SUPEX Council and transacted share of expenses | Approved | 100% |
| Renewed brand usage contract with SK | Approved | |||
| Information system management entrustment with SK | Approved | |||
| Product transactions with SK Life Science, Inc. | Approved | |||
| Service transactions with SK Life Science, Inc. | Approved | |||
| Service transactions with SK Biopharm Tech (Shanghai) | Approved | |||
| Approved quarterly product and service transaction limits for 2021 with SK Biotek | Approved | |||
| Changed the regional development and commercialization license-out contract for Cenobamate in Europe | Approved | |||
| Reporting regarding Arvelle | Reported | |||
| Reorganization and appointment of officers, assignment of duties | Reported | |||
| 10th | 12.29. | Exercised warrant for Arvelle and acquired new shares | Approved | 100% |