Ethical Management
A global pioneer in the development of new drugs
Business Ethics & Practices
In 2024, SK Biopharmaceuticals was recognized for outstanding efforts in the area of business ethics & practices relative to global peers in pharmaceutical industry.
SK Biopharmaceutical promises to make best efforts to create mutual value with all the stakeholders by actively pursuing ethical
management in a sustainable manner.
Pursuit of Ethical Management
Based on SKMS, SK Biopharmaceuticals strives to create greater value for all of our stakeholders, play an instrumental role in social and economic advancement, and contribute to the wellbeing of humanity.
In this context, SK Biopharmaceuticals has established the Code of Ethics that defines essential principles to serve as a guidance in pursuing and maintaining integrity in all of its everyday business decisions and actions.
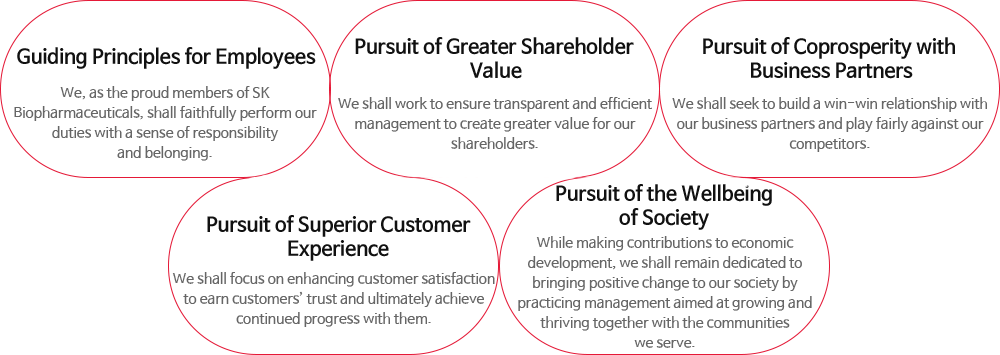
-
Guiding Principles for Employees
We, as the proud members of SK Biopharmaceuticals, shall faithfully perform our duties with a sense of responsibility and belonging.
-
Pursuit of Greater Shareholder Value
We shall work to ensure transparent and efficient management to create greater value for our shareholders.
-
Pursuit of Coprosperity with Business Partners
We shall seek to build a win-win relationship with our business partners and play fairly against our competitors.
-
Pursuit of Superior Customer Experience
We shall focus on enhancing customer satisfaction to earn customers’ trust and ultimately achieve continued progress with them.
-
Pursuit of the Wellbeing of Society
While making contributions to economic development, we shall remain dedicated to bringing positive change to our society by practicing management aimed at growing and thriving together with the communities we serve.

-
Guiding Principles for Employees
We, as the proud members of SK Biopharmaceuticals, shall faithfully perform our duties with a sense of responsibility and belonging.
-
Pursuit of Greater Shareholder Value
We shall work to ensure transparent and efficient management to create greater value for our shareholders.
-
Pursuit of Coprosperity with Business Partners
We shall seek to build a win-win relationship with our business partners and play fairly against our competitors.
-
Pursuit of Superior Customer Experience
We shall focus on enhancing customer satisfaction to earn customers’ trust and ultimately achieve continued progress with them.
-
Pursuit of the Wellbeing of Society
While making contributions to economic development, we shall remain dedicated to bringing positive change to our society by practicing management aimed at growing and thriving together with the communities we serve.
Components of Ethical Management
SK Biopharmaceuticals sets up strategic initiatives based on 10 components in practicing an ethical
management in a sustainable manner.
In particular, as a means to managing internal/external risks and to meeting stakeholders’ needs,
SK Biopharmaceuticals regularly monitors MSCI’s ESG Ratings Methodology as well as key regulatory changes,
continues to search for ways to enhance its operations as per ‘Prevent - Detect - Respond’ cycle and
makes sure that all the employees and stakeholders internalize high ethical values and reflect them in their roles.
-
Governance/Organization
Oversight of the Board & Audit Committee
-
Policies/Processes
Code of Ethics & Anti-Corruption Policy
-
Training
Ethical standards training program
covering all employees
(incl. part-time, contractors) -
Communications
Ethics & Compliance communications
across all operations
-
Risk Assessment/Monitoring
Establishment/Management of
Risk Pool across all operations -
Audit
Audits of all operations every
three years -
Whistleblowing Channel/Investigations
The anonymity secured at all times
(Whistleblower Protection Policy)
-
Reward/Corrective Measures
Transparent process ensured via
formal policies -
Third-Party Management
Ensuring ethical behaviors by
third-parties as per the Third-Party
Code of Ethics -
Oversight of Global Subsidiaries
Customized ethics and
compliance programs
Activities for Promoting Ethics
SK Biopharmaceuticals’ Ethics & Compliance Program operates under the Board and the Audit Committee’s oversight,
and the implementation of key ethics-related activities strictly requires the deliberation and resolution by the Board and the Committee.
SK Biopharmaceuticals runs anti-corruption and business ethics training programs covering all
employees (incl. part-time, contractors) as a means to helping employees internalize ethical principles into daily business conducts.
Also, SK Biopharmaceuticals puts best efforts into setting up risk prevention strategies
through risk pooling, audits of all operations at least once every three years, etc.
Please refer to below items for detailed information.
SK Biopharmaceuticals promises to keep ethics & compliance as the first priority value for all management activities
by complying with global ESG guidelines.
-
[Governance/Organization] Oversight of the Board & Audit Committee
Oversight of the Board & Audit Committee
The implementation of ethics-related activities shall be subject to deliberation and resolution by the Board of Directors, the highest decision-making body of the company, and the Audit Committee under its wing as a means to ensuring the effectiveness of the system.- · Establishment, Amendment of the Code of Ethics, Practice Guidelines, etc.
- · Approving annual audit plan & Providing feedback on interim, final audit reports
- · Deliberation and Resolution on regular, ad-hoc audits
- · Deliberation and Resolution on ethics-related activities & Providing instructions for follow-up arrangements
In 2024, a total of eleven Committee meetings was held, and the Committee reviewed/approved annual audit ∙ ethics management plan as well as mid- to long-term risk management plan.
Below is a list of key agendas reviewed/approved by the Committee. For detailed information, please refer to the link below.- · Annual Audit Plan for 2024
- · Long-term Audit Plan
- · Result of Ethics Program in 2024 and Enhancement Plans
- · Result of Audit & Compliance Program in 2024

-
[Policies/Processes] Code of Ethics & Anti-Corruption Policy
Code of Ethics
As a means to creating values for all stakeholders and acting in a manner benefiting society, SK Biopharmaceuticals has established the Code of Ethics as the standard for decision-making and behavior in all business activities.
The Code of Ethics applies to all employees (incl. part-time, contractors) of SK Biopharmaceuticals and its affiliates, investment companies, and the Code stipulates a wide range of ethics & compliance standards to ensure transparency in conducting business.
In particular, the Company regularly monitors the practicality of the Code to ensure its effectiveness, and the Code gets amended regularly through deliberation and resolution of the Board & the Audit Committee.- · December, 2016 : Establishment of the Code of Ethics
- · July, 2023 : Amendment of the Code of Ethics (1st)

Guidelines for Practicing the Code of Ethics
SK Biopharmaceuticals has established the Guidelines for Practicing the Code of Ethics in order to provide practically applicable guidelines for daily business conducts.
The Guidelines for Practicing the Code of Ethics is structured to provide specific standards for decision-making and behavior based on legitimacy/transparency/rationality criteria so that all employees can abide by the principle of good faith in conducting business.
SK Biopharmaceuticals continues to monitor and amend the Guidelines for Practicing the Code of Ethics.
- · December, 2016 : Establishment of Guidelines for Practicing the Code of Ethics
- · November, 2018 : Amendment of Guidelines for Practicing the Code of Ethics (1st)
- · August, 2021 : Amendment of Guidelines for Practicing the Code of Ethics (2nd)
-
[Training] Programs covering all employees (incl. part-time) and contractors
Ethics & Compliance Training
Ethics training is provided at least once a year to all members (including contract workers). As a result, the level of employees’ ethical awareness has increased significantly with the company achieving 100% training completion rates1) for three consecutive years.
Completion Rate: 2022 (100%) / 2023 (100%) / 2024 (100%)
In addition, SK Biopharmaceuticals strives to transmit ethical values to business partners by providing customized training programs to business partners.
1) Calculated based on the number of active employees
In particular, as a means to enhancing effectiveness of the training program, SK Biopharmaceuticals structures its training curriculum to reflect key risks identified in risk pooling process and conducts surveys afterwards.

-
[Communications] Pledge, Survey, Compliance Letter and Workshop
Ethics Pledge
All employees are required to attest compliance with rules and regulations as a means to internalizing high ethical values. Ethics Survey SK Biopharmaceuticals periodically conducts surveys to gauge employee awareness and to identify any areas for improvement, and all members actively and voluntarily participate in the surveys.- · Participation Rate: 2022 (85%) / 2023 (90%) / 2024 (91%)

-
[Risk Assessment/Monitoring] Risk Management across all operations
Corporate Risk Pool
SK Biopharmaceuticals runs an annual risk assessment/monitoring program
as an effort to effectively control and oversee corporate-level risks including but not limited to corruption risks.SK Biopharmaceuticals annually updates the risk pool
and actively make use of such risk pool in conducting audits of all operations every three years.
[Risk Prioritization]
In the process of creating a risk pool, SK Biopharmaceuticals evaluates the likelihood and consequences associated with each risk and prioritize them.
Once corporate functions are classified into 6 areas and 43 sub-sectors,
risks are assessed and ranked based on their potential impact and likelihood of occurrence.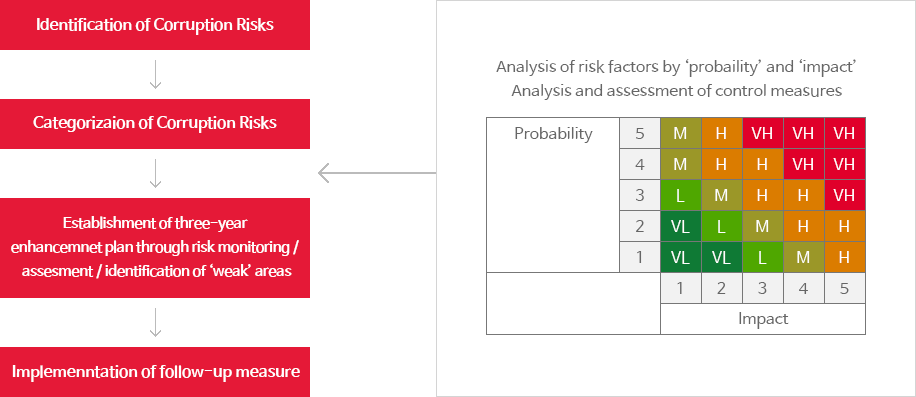 <Risk Prioritization Process>
<Risk Prioritization Process>
-
[Audit] Audits of all operations every three years
Deficiency Review of Internal Controls
SK Biopharmaceuticals conducts an annual risk review/audit on key operations covering HR management, cost management, procurement, marketing/sales management, investment management, etc. An annual risk review/audit is conducted by the audit team as a means to proactively mitigating ethics and compliance risks.
The result of such review/audit is reported to the Board and the Audit Committee on a timely manner, and risk mitigation activities are implemented under the supervision of the Board and the Committee.
Since 2023, SK Biopharmaceuticals has expanded the scope of review to include foreign subsidiaries. In addition, SK Life Science conducts regular reviews on sales & marketing activities from regulatory compliance perspective.
<Deficiency Review History>
Area of Review 2022 2023 2024 HR Management ○ ○ ○ Expense Management ○ ○ ○ Vendor Management ○ ○ ○ Sales Management ○ ○ ○ Investment Management ○ ○ ○ Fund Management, etc. ○ ○ ○ Audits of all operations (Every three years)
SK Biopharmaceutical conducts audits of all operations at least once every three years according to a company-wide risk pool.
[Audits of all operations every three years]
The audit team conducts audits of all operations every three years, and the Board and the Audit Committee review/approve a three-year audit plan. After the approval, the audit team provides updates to the Board and the Committee by providing progress/final/follow-up reports.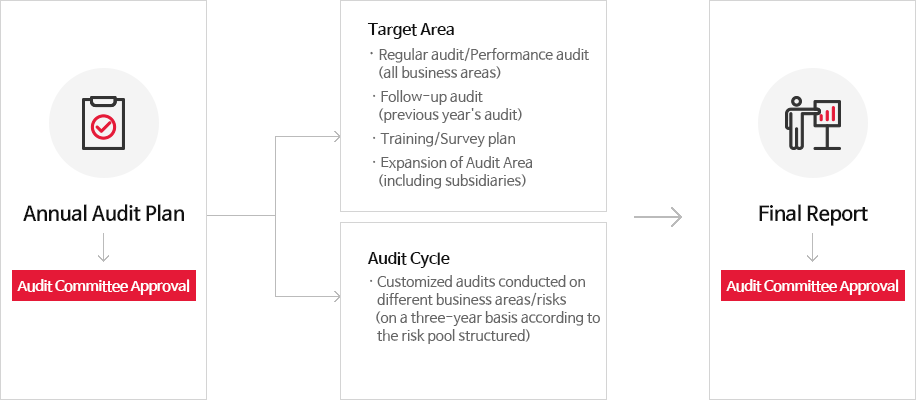
< 3-year-cycle Company-Wide (including domestic/international subsdiary company)Audit System>
[Audit History]
Since 2023, SK Biopharmaceuticals has also expanded the scope of audit to include foreign subsidiaries. In particular, the company conducted a large-scale audit of all corporate functions in 2024.
<Audit History>
Function 2022 2023 2024 R&D ○ Management Support ○ ○ ○ Manufacturing/Quality Management ○ ○ ○ Business/Investment Management ○ ○ ○ Sales & Marketing ○ Subsidiary ○ ○ -
[Third-Party Management] Third-Party Code of Ethics
Third-Party Code of Ethics
SK Biopharmaceuticals strives for the highest tier of global ethics/compliance leadership, and SK Biopharmaceuticals recommends all third-parties to adopt an ethics management structure by establishing the third-party code of ethics. The third-party code of ethics encompasses a wide range of business ethics, including but not limited to Labor & human rights, Safety & Health, Environment and Ethics. SK Biopharmaceuticals recommends all third-parties to foster a sustainable business model by maintaining ethics management system and comply with rules and regulations. Partner Code of Ethics
Third-Party Pledge of Compliance & Ethical Management Support
All business partners are regularly asked to attest their compliance and to participate in the surveys every year. To prevent corruption and practice ethical management for major business partners, we conduct ethical management support activities such as providing ethics training, sharing newsletters and ethical management infrastructure, every year. -
[Whistleblowing Channel/Investigations] Whistleblower Protection Policy
Whistleblower Protection Policy
Whistleblowing system at SK Biopharmaceuticals is operated properly so that any members of stakeholders are free to voice their concerns at any time. In particular, SK Biopharmaceuticals maintains Whistleblower Protection Program as a means to protecting the confidentiality of the whistleblower and whistleblowing report, and any whistleblower shall not and will not be subject to any forms of retaliation. For detailed information, please refer to the links below. Whistleblowing Channel SK Biopharmaceuticals Whistleblowing Channel SK Group Whistleblowing Channel
-
[Reward/Corrective Measures] Transparent Process
Reward∙Disciplinary Policy
By maintaining the reward∙disciplinary policy which outlines clear-cut reward∙disciplinary standards, SK Biopharmaceuticals strives to enhance its risk management capacity. In particular, SK Biopharmaceuticals puts best efforts in making sure that all decisions made are unbiased and are based on all available information as a means to promoting fairness and transparency especially in disciplinary and grievance procedures. Every reward∙disciplinary decision is subject to deliberation and resolution by the HR committee and the Reward∙disciplinary committee, and, in case of any issues, the audit team is responsible for submitting a proposal to the HR committee after thorough investigations.
-
[Oversight of Global Subsidiaries] Customized Ethics & Compliance Programs
Expansion of Ethical Management
SK Life Science, a wholly-owned subsidiary of SK Biopharmaceuticals, maintains its own anti-corruption policy, Code of Ethics and the Third-Party Code of Ethics and runs a variety of ethics & compliance programs. Recently, SK Biopharmaceuticals successfully enhanced the level of ethics & compliance program alignment with SK Life Science’s across a variety of subjects, including risk pooling, audit, training. SK Biopharmaceuticals keeps striving to promote such alignment to drive accountability. For detailed information on ethics & compliance program at SK Life Science, please refer to the link below. SK Life Science Ethics & Compliance Program
